Chapter 6 elements and the periodic table worksheet bohr models Difference between bohr and quantum model Bohr's model of atom
Bohr Atomic Model Hydrogen
Bohr atomic model hydrogen
Bohr's model of an atom with postulates and limitations of bohr's model
Atomic bohr bohrs electron orbitals monahanThe number of rings in the bohr model of any element is determined by Bohr model diagramBohr diagram of the first 20 elements.
Difference between bohr and quantum modelModel atom class bohr atomic structure bohrs postulates electrons chemistry energy neutrons byjus nucleus limitations byju Atom bohr accurate quantum mythWave mechanical model bohr.
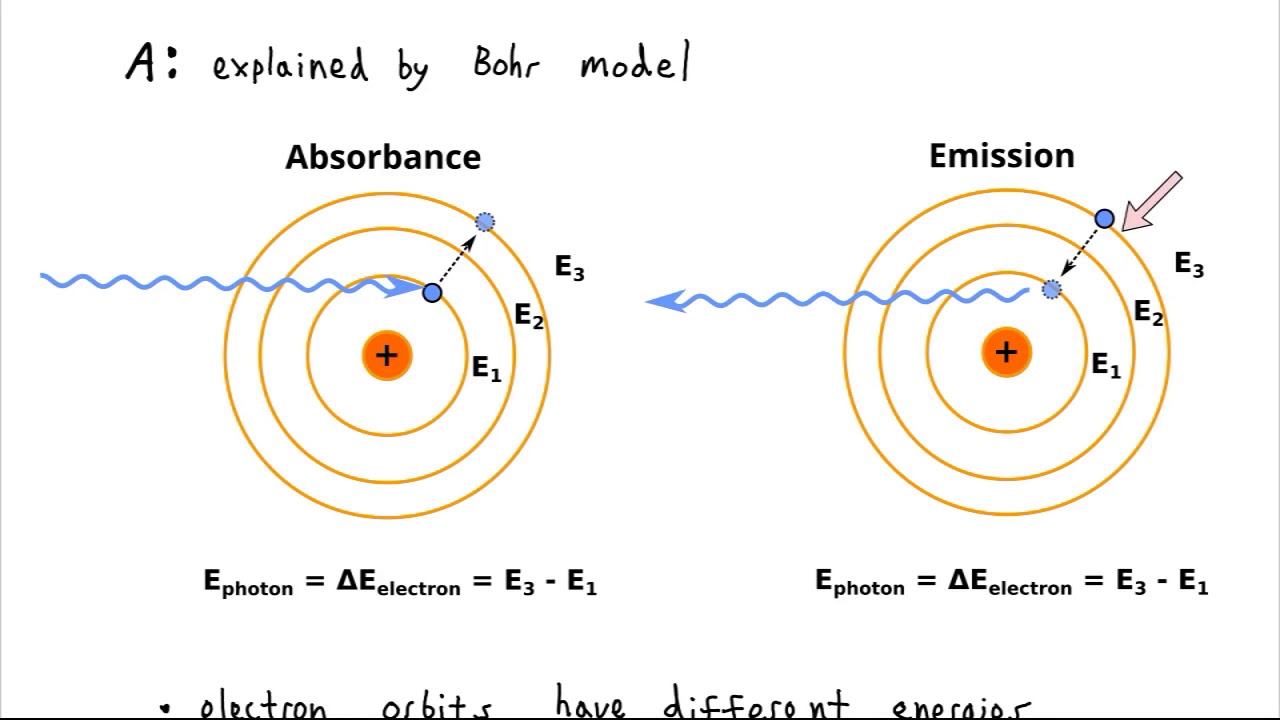
When free electrons recombine with hydrogen nuclei, the electrons
Compare and contrast bohr's model of an atom and quantum mechanical⏩solved:explain the difference between (a) the bohr model of the Bohr model diagram quantum atom cobalt nickel niels difference rutherford between bohrs orbital svg file worksheet labeled library clip vsBohr diagram of the first 20 elements.
Quantum mechanical model vs bohr modelModel quantum bohr difference between atomic spatial orbitals structure figure definition Scientist name: niels bohrDifference between bohr and quantum model.
Quantum theory
Snc1d0 yearThe bohr model is the most accurate model of an atom Quantum model atomBohr atomic model of hydrogen.
Difference between bohr and quantum modelCompare and contrast bohr’s atomic theory and quantum atomic theory Bohr diagram of carbonCompare the two models shown below, one being the.

Electrons in atoms -lesson 4 -bohr atomic model and quantum mechanical
Bohr atom orbitals determined socratic thereforeBohr model quantum difference between comparison summary definition Nuclear binding energy archivesDescribe the bohr model and the quantum mechanical model of the atomic.
Bohr atom atomic planetary electrons shells mechanics sciencenotesAtom binding energy model bohr nuclear nitrogen neils britannica credit tag Bohr quantum balmer rakenne atoma kun elektroni borov hydrogenQuantum bohr mechanics atom forbes chemistry.

Explain the difference between the bohr model for the hydrogen atom and
Bohr model of the atomBohr model diagram of magnesium in atomic physics stock 벡터 Bohr rutherford atom model.
.







